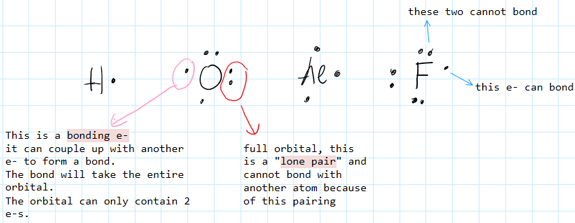
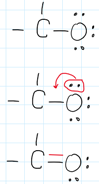What is a Lone Pair?
A lone pair fills the entire orbital and prevents bonding.
Electrons need to be alone in their orbitals to permit the pairing with another single electron (alone in its own orbital as well) of another atom which is the bonding.
Lone pairs are helpful when trying to add a double (or triple) bond. This is when the lone pair can be shared across two separte atoms. This can happen when one of the bonding atom does not have enough electrons to complete an octet, as it is with the Carbon atom below. This way both participating atoms can have a full octet.
In this example C has only 6e-. When the red bond below between C and O is shared, both C and O is surrounded by an octet.
(remember that each bond is equal to two electrons when counting electrons for the octet)