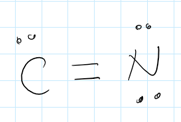
Calculate the FC of an atom in a Lewis structure.
FC(atom) = the number of valence electrons - "sticks" - "rocks"
sticks = bonds,
rocks = electrons in lone pairs.
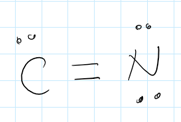
FC(C) = valence of C - sticks - rocks = 4 - 2 - 2 = 0
FC(N) = valence of N - sticks - rocks = 5 - 2 - 4 = -1